
Optimized dosing regimens for the combinations of sulphonamides and trimethoprim in veterinary medicine (SulTAn)
To fill the gaps related to the pharmacokinetics of Trimethoprim and Sulphonamides, and to the pharmacodynamics (PD) of their interaction on veterinary pathogens across multiple animal species.

Challenge
Recent decades have seen a reduction in options to combat the increasing incidence of drug resistant infections in human medicine. Solutions to combat Antimicrobial resistance (AMR) require a one Health approach between animals, humans and their environment on a local, national and global scale.
Sulphonamides (S) and trimethoprim (TMP) are broad spectrum AMD, grouped in category D, and are authorized in all domestic species. It is anticipated that their relative use will increase in the coming years all over Europe. As these AMDs were approved in the 80s their rational for dose selection is based on limited pharmacokinetic (PK) / pharmacodynamic (PD) data. Registered dosing regimens for these AMDs are most probably suboptimal and may require a critical re-evaluation.
In fact, suboptimal dosing regimens may lead to higher consumption of AMD due to (i) an inefficacious first-line treatment with TMP and S, followed by (ii) recourse to a second-line AMD that is often more critical for human health.
Furthermore, Sulfonamides and TMP are normally combined in formulations to give synergistic effects that are bactericidal for pathogenic bacteria. Nearly all marketed veterinary formulations the ratio is 1:5 (TMP:S), an extrapolation of data obtained for the sulfamethoxazole (SMX): TMP combination in humans, which results in a ratio between the maximum plasma concentrations (Cmax) of 1:19. In veterinary species this ratio has not been tested, and needs optimisation, to account for:
- The wide range of veterinary pathogenic bacteria
- PK differences between animal species
- PK differences between different sulphonamides (sulfamethoxazole, sulfadiazine, sulfadimethoxine).
Solution
This project will focus on the combination of different S with TMP in veterinary medicine and will aim at determining the needed adjustments or revisions to optimize TMPS dosage regimens in domestic animal species.
These include dogs, horses, pigs, poultry, cattle, sheep/goats, and fish. Covering a range of pathogenic bacteria identified as the most frequently occurring cause of infection in each species.
This objective will be reached by:
- characterizing the effects of the drugs alone and their interaction in combination on veterinary pathogens (MIC determination, checkerboard assays, and PD studies);
- describing the fate of TMPS in the different animal species (PK studies);
- Advanced PK/PD modelling.
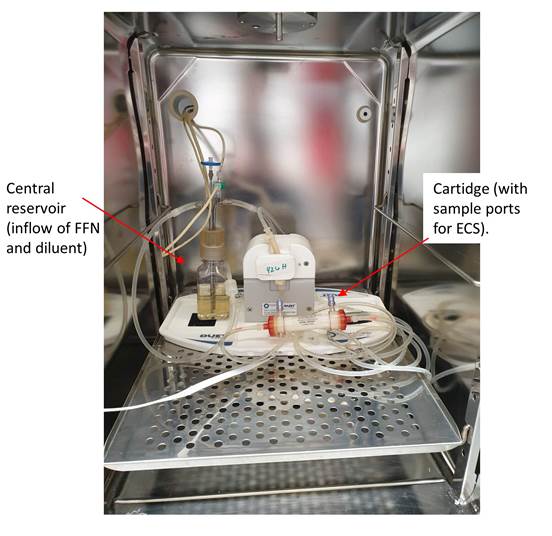
The antibacterial effects of the optimized dosing regimens proposed at the end of the project will be ultimately verified by PK/PD in vitro studies in hollow-fibre infection models (HFIM). The HFIM provides a dynamic 2-compartment system that accurately emulates the host PK profile for a specific dose (or doses) and allows the bacteriological effect to be monitored over-time.

Impact
Antimicrobial resistance is a truly global problem, and therefore it affects the whole world at human, animal and environmental levels.
As well as offering a benefit to animal owners in the form of better therapeutic options for the health and welfare of the animals.
The prudent and responsible use of AMD in veterinary medicine aims at guaranteeing benefits for animal health and welfare while limiting the impacts on human and environment health. Responsible use avoids useless treatments on resistant bacteria and ensures efficacy on susceptible pathogens that require treatment with AMD. By strictly limiting the use of AMD to the cases for which efficacy is expected, the pressure for bacterial resistance selection in animals and the quantities of the drug ultimately excreted in the environment are limited. Any reduction in the excretion of resistant bacteria and antibiotic residues by animals can help reduce the risk of transmission of resistant bacteria to the human reservoir.
For human health, an additional benefit of the optimization of first-line therapy, such as TMPS, in veterinary medicine is that the recourse to second-line antibiotics, often classified as critically important for human medicine, can be dramatically decreased.
Furthermore, the developed scientific approach on PK/PD modelling for drug combinations, taking advantages of data generated under dynamic conditions (using the HFS), will also rapidly benefit other drug combinations in veterinary as well as in human medicine.
Partners
The Joint Programming Initiative on Antimicrobial Resistance (JPIAMR)

